With the recent progress in the field of immuno-oncology, harnessing the power of the immune system to fight cancer has become one of the pillars of cancer care. However, there is still much to be done, given that only a subset of patients are responsive to a new class of drugs called immune checkpoint inhibitors (ICI). A better understanding of the cancer immune biology and the repressive mechanisms existent in the tumor microenvironment will certainly provide new clues on how to effectively target tumors in a more comprehensive and tailored way. Among the immune cells populating solid tumors, myeloid cells represent a large part and are known to play important tumor-promoting roles by their immunosuppressive effects on T-cell activation and promotion of tumor growth and metastasis. Previous studies have shown that the presence of myeloid cells, such as tumor-infiltrating neutrophils, macrophages and myeloid-derived suppressor cells (MDSCs) is associated with poor prognosis and ICI resistance in patients with various tumor types. Nevertheless, given their highly plastic phenotype, myeloid cells are able to exert a dual role on tumor growth depending on the local tissue environment. For instance, macrophages undergo a series of functional reprogramming depending on the stimuli they receive, as described by two different polarization states, M1 and M2. M1 macrophages represent pro-inflammatory cells that are able to mediate resistance to pathogens and promote antitumor immunity, while M2 macrophages are characterized by their tissue-remodeling, immune regulatory and tumor-promoting activity (Figure 1).
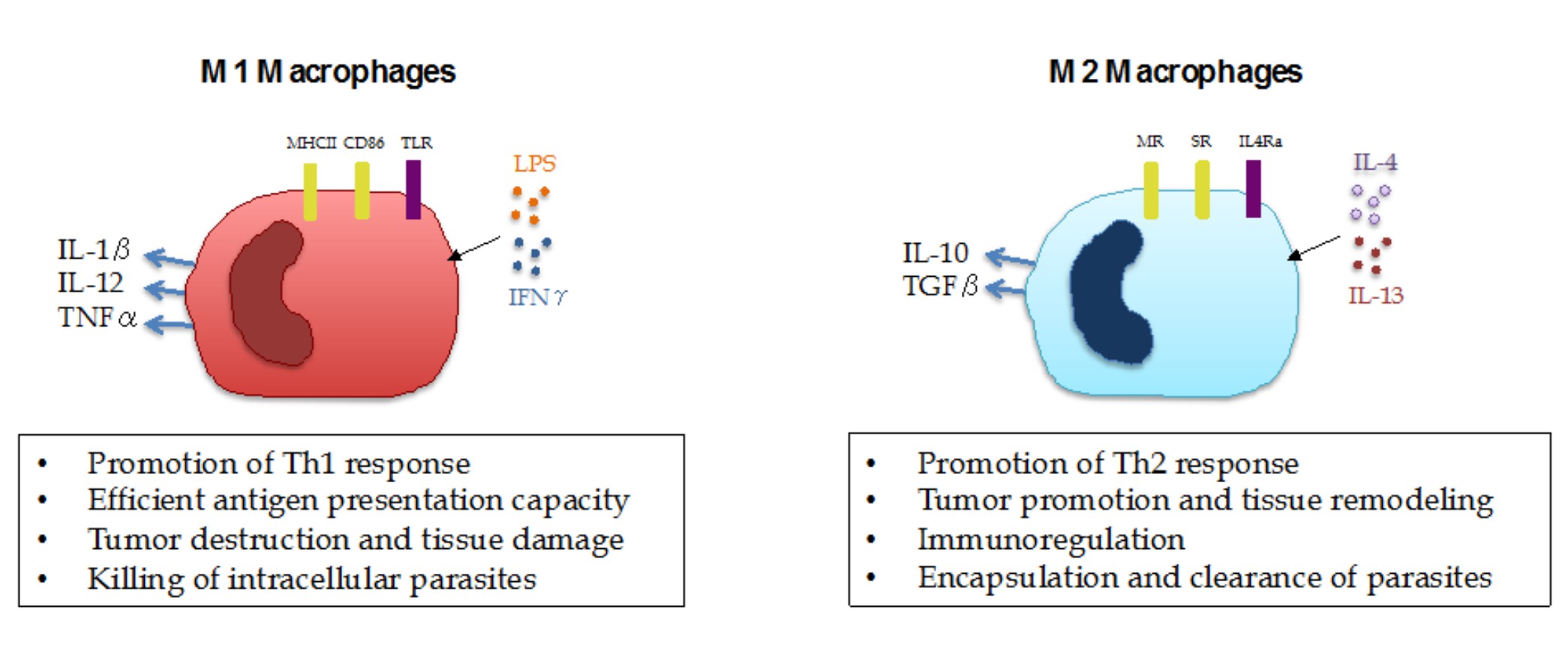
With that, one of the most promising avenues of immuno-oncology research is figuring out how to skew the activation of myeloid cells from an immunosuppressive state towards a pro-inflammatory, antitumor phenotype in order to facilitate ICI response. Two recent publications in Nature by De Henau and collaborators and Kaneda and collaborators have described the role of a signaling protein called PI3K gamma (PI3Kγ) as a key molecular switch that controls the immunosuppressive state of myeloid cells. These studies show that this pathway is highly active in suppressive myeloid cells and that its selective inactivation promotes a functional switch in myeloid cells, leading to an immunostimulatory transcriptional program that restores T-cell activation and cytotoxicity. Importantly, De Henau and collaborators showed that PI3Kγ inhibition only works on myeloid-enriched tumors and that it synergizes with ICB therapy (anti-PD-1 and anti-CTLA-4) to promote tumor regression and increased survival in mouse models of cancer. These findings introduce opportunities for new, more tailored combination therapies based on the patient's tumor-immune landscape.
This is certainly good news for cancer patients, and it is important to point out that the use of a PI3Kγ inhibitor is currently being tested in a Phase I clinical trial (clinicaltrials.gov identifier NCT02637531). These studies represent great examples of a successful bench to bedside research enterprise. Hopefully, there will be many more to come in the years ahead.
Further reading on this topic:
Sica A and Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas Journal of Clinical Investigation 122: 787-795
 Medical Research
Medical Research
Responses