Cancer Immunotherapy and the Microbiome: It's Time to Follow Our Guts
By Luis Felipe Campesato in Medical Research |
Medical Research | The human microbiota, or the microbiome, is the ensemble of bacteria and other microorganisms (archaea, fungi, protozoa, as well as human, fungal, bacterial and protozoan viruses) that inhabit the epithelial barrier surfaces of our body. The microbiota in the gut, for instance, comprises approximately 3 x 1013 bacterial cells that mostly exhibit commensalism with the host. Changes in the composition of the microbiota have been connected to many aspects of host physiology as well as human diseases, including those related to metabolism, neurological and cognitive functions, inflammation and immunity. Disrupting the microbiome repertoire of the gut, which is also referred to as “intestinal dysbiosis,” has been associated with a variety of chronic inflammatory disorders, such as inflammatory bowel disease, irritable bowel syndrome as well as extra-intestinal disorders, including allergy, asthma and obesity. Much of the work, though, remains associative; relatively few microbe–host interactions have been defined at the level of molecular mechanisms.
The local microbiota, such as the one found in the skin, oropharynx, and respiratory, digestive, and urogenital tracts, is known to affect the functions of the epithelial barrier on which it resides, including immunity-related aspects. By its interaction with epithelial and stromal cells, a healthy gut microbiota is able to regulate barrier functions, preventing infestation by pathogens and overgrowth of pathobionts, controlling the metabolism of indigestible dietary fibres and the synthesis of vitamins, and regulating metabolism in general. However, the mechanisms by which local microbiota exert effects systemically, such as the ones modulating host inflammation and immunity, are much less understood than those mediating the local effects.
One of the potential contributions of gut bacteria to host biology is the circulating pool of bacterially derived metabolites, which can reach or exceed concentrations achieved by a typical drug dose (10 μM–1 mM). In many cases, these compounds exhibit specificity by engaging receptors thereby altering host biology at a systemic level. Recently, a study showed that the gut symbiont Clostridium sporogenes generates aromatic amino acid metabolites (from tryptophan, phenylalanine and tyrosine) of which nine are known to accumulate in host plasma and affect intestinal permeability and systemic immunity. The gut microbiota has also been shown to drive the maturation and function of different immune cell types. Several bacterial taxa shape the differentiation of naïve T cells: segmented filamentous bacteria (SFB) bias T helper cells towards a Th17 phenotype while Clostridia clusters IV and XIVa promote regulatory T cells (T-Regs) differentiation. Given the interconnected nature of the microbiota and the immune system, it is plausible that the microbiota has the ability to influence immune-mediated disorders, such as cancer.
In fact, a number of recent studies proposed a link between the gut microbiota and cancer. For instance, epidemiological studies link intra-abdominal infections, the use of antibiotics, or both, to an increased incidence of colorectal cancer. Abrogating or specifically altering the composition of the gut microbiota has been shown to influence the incidence and progression of colorectal carcinoma in both genetic and carcinogen-induced models of tumorigenesis. The ways in which microbes and the microbiota modulate carcinogenesis have been proposed to fall into three broad categories:
- Their ability to alter the balance of host cell proliferation and death
- Guiding immune system function
- Influencing metabolism of host-produced factors, ingested foodstuffs, and pharmaceutical drugs.
Importantly, alteration in the composition of intestinal bacteria has been shown to also influence the response against different anticancer agents, including chemotherapy, radiotherapy and, most recently, immunotherapy.
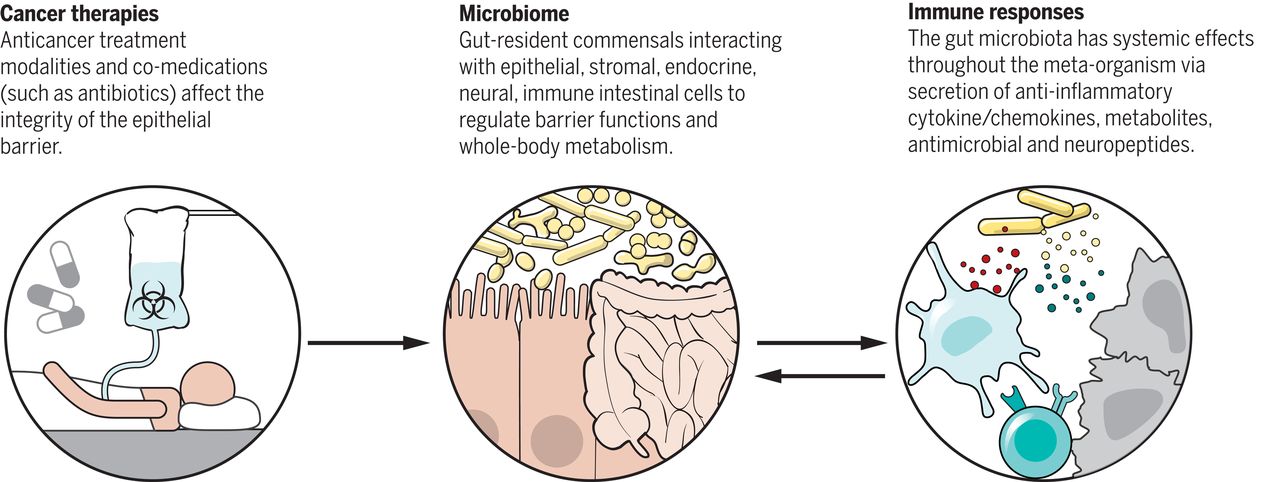
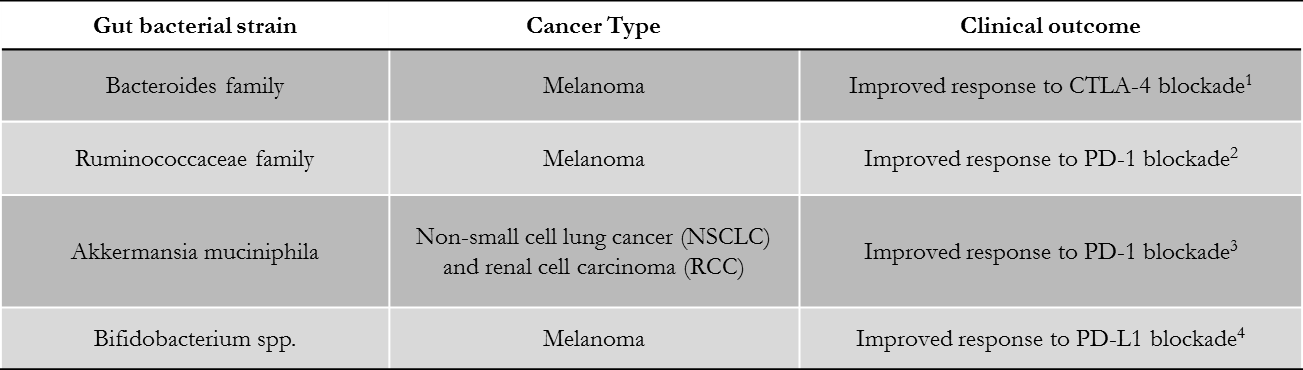
In 2015, a study from Zitvogel et al. found that the antitumor effects of CTLA-4 blockade depend on the presence of distinct Bacteroides species in the gut of cancer patients. The authors described that upon CTLA-4 blockade, intraepithelial lymphocytes damage intestinal epithelial cells, stimulating the accumulation of Bacteroides species, which are able to activate IL-12–producing dendritic cells (DCs) and T helper 1 (TH1) immune responses. A more recent study, in 2018, linked an abundance of bacteria of the Ruminococcaceae family with response to anti-PD-1/PD-L1 therapy, this time in melanoma patients. In each case, therapeutic efficacy was reduced when the gut microbiota was absent or manipulated. Corroborating these findings, several independent retrospective analyses in human cohorts of metastatic lung, kidney, and bladder cancer patients indicated the deleterious role of different classes of antibiotics taken around the initiation of PD1/PDL-1 blockade. In addition, bacterial strains generally associated with health (such as Clostridiales, Ruminococcaceae, Faecalibacterium spp., Akkermansia muciniphila, B. fragilis, and Bifidobacteria) or immunogenicity (Enterococci, Collinsella, and Alistipes) were abundantly detected in patients who were responsive to anti-PD-1 therapy.
Lastly, but not less importantly, new evidence links the abundance of certain gut microbes with toxicity-related events observed in some patients after immune-checkpoint blockade (CTLA-4 and PD-1 blockade), such as severe colitis.
Although most of the studies in the field remain of associative nature, there are hypothetical mechanisms that may explain a causal connection between gut flora and development of anticancer immunity:
- Through the stimulation of T cell responses against microbial antigens, which either provide help for tumor-specific immune responses, or may cross-react against tumor-specific antigens
- Through engagement of pattern recognition receptors that act as adjuvants and mediate pro-immune or anti-inflammatory effects
- Through small microbiota-derived metabolites that mediate systemic effects on the host.
Nevertheless, the intersection between the microbiome and cancer immunotherapy is still a very young field of research and, as new findings are reported and more patients are enrolled in clinical studies, investigators will hopefully interrogate these questions in a more systematic and thorough manner. A lot still remains to be learned. The link between the efficacy of cancer immunotherapy and the gut microbiome has undoubtedly become one of the most exciting and promising areas of investigation for cancer care over the past decade. A deeper understanding of microbe–host interactions will potentially allow us to develop better anticancer therapies and reduce systemic toxicity. Targeting the microbiota in cancer and other diseases is likely to become one of the next frontiers for precision and personalized medicine.
Table references:
- Vetizoy M, et al. (2015) Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350: 1079-84
- Gopalakrishnan V, et al. (2018) Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359: 97-103
- Routy B, et al. (2018) Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359: 91-97
- Sivan A, et al. (2015) Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350: 1084-9
Further reading on this topic:
- Zitvogel L, Ma Y, Raoult D, Kroemer G, and Gajewski TF (2018) The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 359: 1366-1370
- Roy S and Trincheri G (2017) Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer 17: 271-285
Responses