Three Centuries of Hunting for Ice Age Mummies and the Prospect of De-Extinction
By Olga Potapova, Beth Shapiro, and Albert Protopopov in Paleobiology |
Paleobiology | Knowledge of prehistoric “monsters” that lived underground is rooted in centuries of oral history, folklore, and languages of Siberian tribes. These tales were collected by Russian Cossacks sent in the 16th century by Ivan the Terrible to conquest Siberia, and continued to be collected and shared throughout the next three centuries by European merchants and diplomats travelling to the East and by Chinese traders of Siberian mammoth ivory. Native members of the Evenks (Tunguz), Nenets (Samoyeds), Bashkirs (Ostyaks), Selkups, Evens (Lamuts), Yukagi (Oduls), Yakuts (Sakha), Chukchi, and other tribes often encountered frozen remains, tusks, and carcasses of large (but never alive) animals, thawing out from the ground. According to this lore, these animals, called by different names, were mostly the woolly mammoths that lived underground, in the wild, or in rivers and lakes–and avoided direct contact with humans. The widespread belief that woolly mammoths and other “underground creatures” inhabited isolated territories in Siberia lasted until the beginning of the 19th century. Such beliefs even extended beyond Siberia, with tales of similar creatures inhabiting the unexplored lands of Louisiana in the United States. Only a few scientists of the time questioned these beliefs that were even supported by the 3rd President of the United States, Thomas Jefferson. Still, no one at the time had yet considered the idea of scientific “resurrection” of the “underground monsters”. At that time, “hunting” for frozen extinct animals with the goal to recover the valuable scientific data stored in their tissues and cells had not yet begun either. However, the door to those possibilities would soon be opened wide.
Early finds
A Special Decree was made by the Russian tsar Peter the Great in 1718, which promised rewards for collecting curiosities and rarities. This decree inspired searches for unusual objects, and especially for frozen mummies. At the time, in the 18th century, profound superstitious native beliefs understood that encountering the “underground creatures” would bring bad luck, disease, and even death to the family of the mummy’s discoverer, and sometimes the whole village. Reports of mummies from Siberia were therefore rare. Nonetheless, the 18th century marked the first salvage of frozen body remnants of a large extinct species. In 1771, a woolly rhinoceros was discovered in the Vilyuy River in Eastern Siberia. The head, two legs and hide were delivered to the Kunstkamera (Peter the Great’s Museum of rarities) in St. Petersburg, and served as the basis for the first formal description of this extinct species (Fig. 1).

In the following two centuries, the Imperial Academy of Sciences and its successor, USSR Academy of Sciences, announced monetary rewards for skeletons and carcasses of large fossil animals (Fig. 2) in order to satisfy the growing demand for museum exhibits and research.

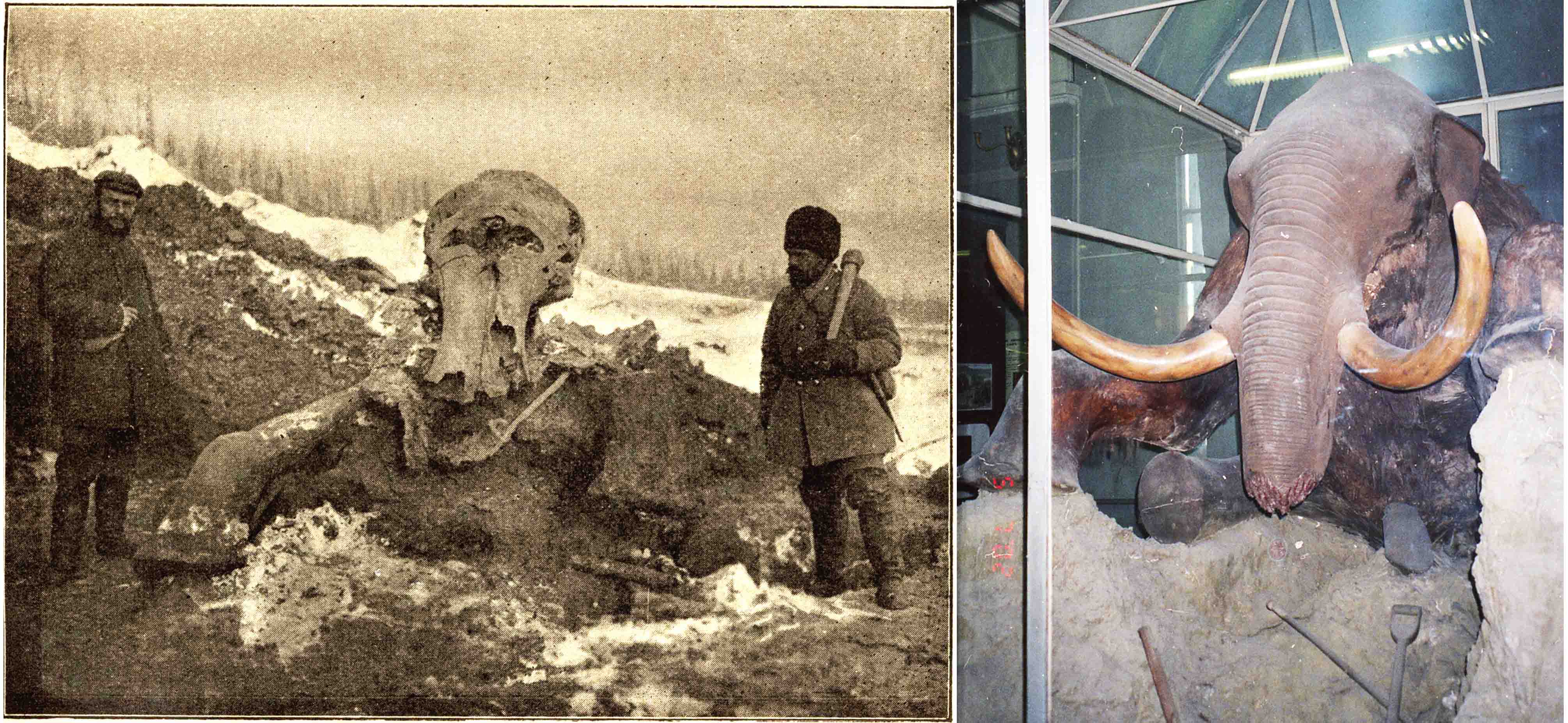
As a result, between 1805 and 1918, 30 frozen carcasses of woolly mammoths and woolly rhinoceroses were reported from Siberia (Tolmachoff, 1929). Nevertheless, none of these carcasses discovered in remote areas could be recovered, and only a few parts of their remnants, such as hair, bones or tusks ever made it to museums. Among the salvaged remains were the head and foot of the second complete woolly rhino, which was found in 1877 in the Yana River basin. It was not until the end of the 19th and the beginning of the 20th century that expeditions sent by the Imperial Academy of Sciences excavated and recovered complete skeletons, skin, some internal organs, and soft tissues from the frozen bodies of the Adam’s mammoth (1900) and Berezovka mammoth (1901)(Fig. 3) that were discovered in the East Siberian Arctic.
Many of those early finds were lost to science because expeditions to salvage the specimens were never organized or arrived too late at the site. Among those lost were magnificent complete bodies of mammoths buried in standing up positions, such as the Sarychev mammoth discovered in 1787 in the Alazeya River basin, the Rozhin mammoth found in 1839 in the Indigirka basin, and the complete White Horse (extinct Pleistocene wild horse) reported in the late 19th century from the lower Yana River. Many other salvage expeditions arrived too late, only to find that the reported frozen carcasses, such as that of the Khitrovo mammoth from the Kolyma River basin (1854), the Stschelkanov mammoth from northern Yakutia (1948), and many others had collapsed into rivers and lakes from thawing shores.
Still other newly exposed and thawed carcasses were lost in a different way. Often, thawing mummies turned into a commodity for native people living in remote areas, who used the flesh for feeding their dogs or used its fat to lubricate small boats. With few exceptions, “tuskers” chopped off and sold tusks from most of the discovered frozen bodies. Expeditions to the mummies were sometimes plainly sabotaged by local officials, in order to avoid headaches and additional expenses. One of the best specimens ever found, the frozen body of the Mostak mammoth, which was discovered in 1857 in the mouth of the Lena River, was chopped up and thrown in the river following a local official order. Prior to being chopped up, of course, the mammoth’s tusks were broken off and sold for cash.
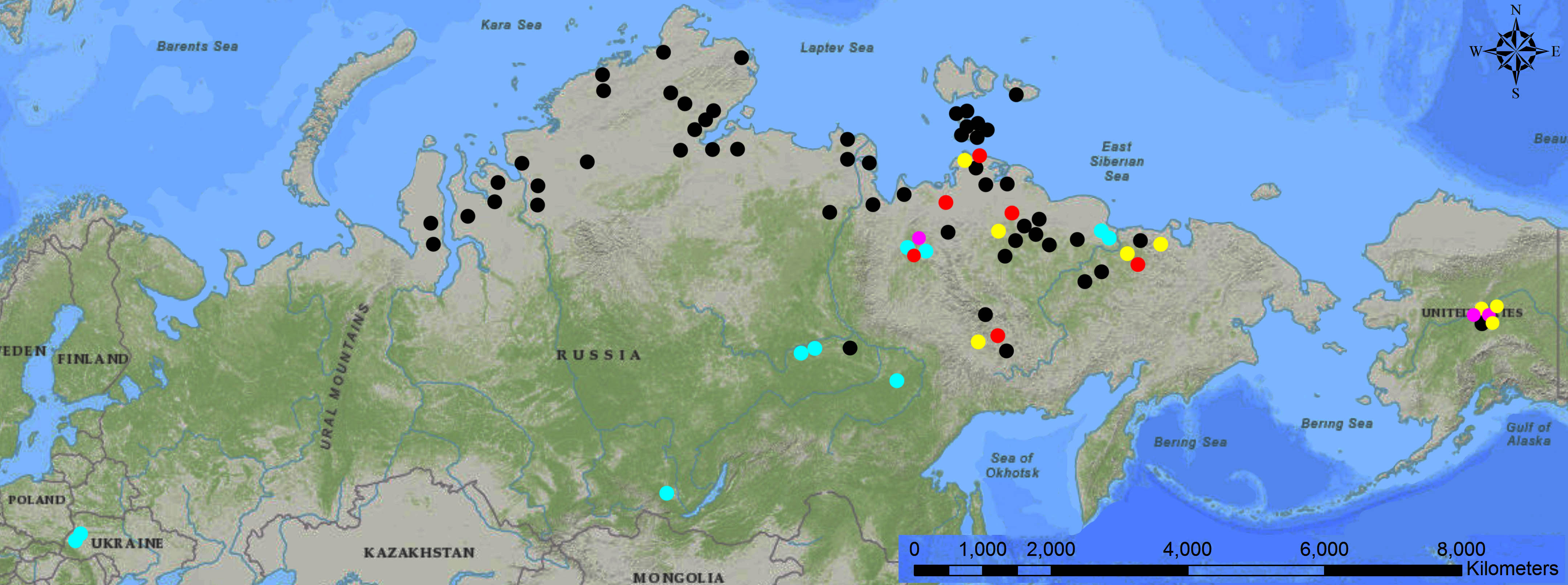
In Siberia, finds of frozen mummies began to be managed again only in the second half of the 20th century, following the economic recovery of the Soviet Union after the 1917 Revolution, the two World Wars, and resumption of Arctic exploration. Around the second half of the 20th century, Russian and American industrial gold mining operations in remote areas of the north had a significant impact on the recovery of Ice Age mummies. Mining activities led to the discovery of the partial body of “Effie” the baby woolly mammoth (1948, Fig. 4) and the mostly complete body of “Blue Babe” the steppe bison (1990) in Alaska, and the Selerikan horse (1968) and “Dima” the baby woolly mammoth (1977) in Eastern Siberia.

The end of the 20th century through the beginning of the 21st century was marked by a new (and still lasting) wave of discoveries of frozen bodies by private prospectors of mammoth tusks driven by the growing demand for ivory in China and the widespread licensing of private “tusk hunting” companies in Russia (Fig. 5).
Initial scientific analyses
During the first 150 years of collecting the mummies and their parts, the majority of scientific research was devoted to descriptions of exterior morphology and comparisons with other extinct species. However, as the mummies were studied, the pinkish color of their tissues and the “bloody” look of the flesh, which was readily eaten by dogs, attracted the attention of scientists. As early as the 18th century, it was clear to scientists that different types of soft tissues, and not just the skin and bones, were preserved in mummies.
The first microscopic studies of Ice Age animal tissues were performed by V. Glebov in 1846. Using the Mochulsky (Trofimov) mammoth he identified muscle, connective and fat tissues, as well as tendons, brain tissue, and even the cortical and medullar components of the hair. This very first scientific analysis was soon followed by more specific histological and cytological analyses of the Vilyuy rhino’s muscles, connective tissues and cartilage performed by the Russian Academician J. F. Brandt in 1849. This study in turn was followed by examination of the Berezovka mammoth’s tongue and leg muscles, and a biochemical analysis of its “blood” carried out by Russian zoologists V. Zalenskiy, N. Maliev, and Byalynitskiy-Birulya in 1849-1909. For both the Vilyuy and Berezovka specimens the investigators noted the good preservation of the collagen fibers and the striated fiber pattern of the muscle tissues. They also stated, however, the almost complete deterioration of all cellular structures in the examined tissues.
Modern microscopic analyses
Light microscopy studies of the eye muscles from the 21,000 year old Effie baby mammoth were performed in the 1970s. From 1977-1979, examinations of the muscle, connective tissue, blood vessels, skin, fat and internal organs of the 42,000 year old Shandrin mammoth, 53,000 year old Khatanga mammoth, the 38,000 year old Selerikan horse, and the 30,000 year old Mylakhchin bison were carried out. These efforts were successful in identifying striated muscle tissues, but with few exceptions neither of the studies revealed preserved cellular structures. There were claims that some of the cells identified from the uterus tissue of the Mylakhchin bison and from the liver of the Selerikan horse contained a nucleus or nuclear fragments, but these claims have never been subjected to verification.
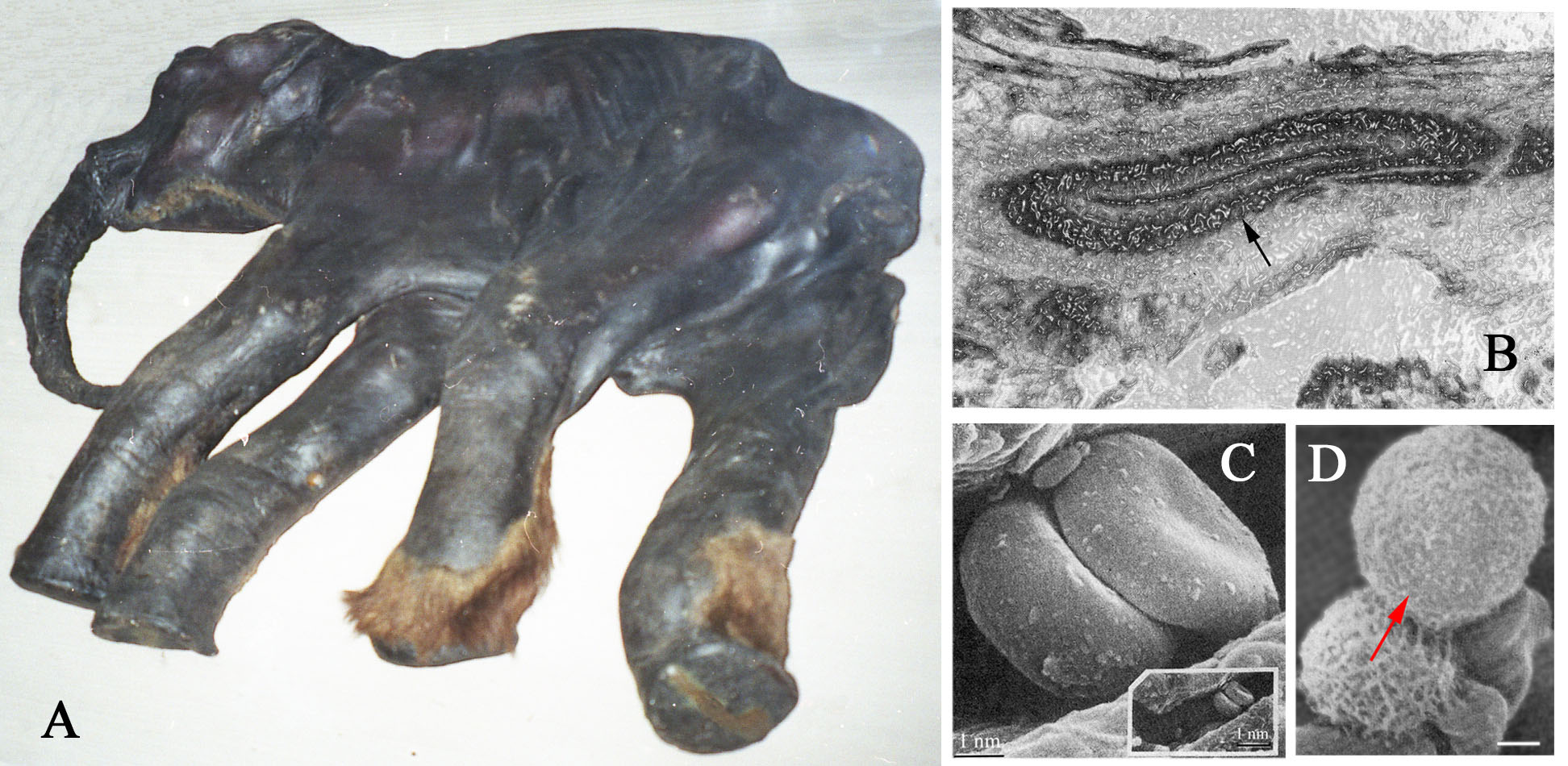
In the late 1970s, frozen samples of soft tissues from the almost complete Dima baby mammoth (Fig. 6A) were examined with a scanning electron microscope (SEM) and light microscope. Studies of the skin revealed blood vessels with individual layers being discerned in the vessel walls (Fig. 6B) and veins that retained clearly visible valves. The epidermis, hair follicles and connective tissue cells in the skin were completely deteriorated. The collagen fibers were easily detectable in the main arteries and veins, as well as in the blood vessels in most of the investigated organs, which included the brain membranes, tongue, esophagus, lungs, heart, liver, pancreas, kidneys, colon, and a testicle. SEM images of the muscle tissues showed that the muscle fibers grouped in bundles seemingly preserving remnants of myosin and actin molecules. Shapes or outlines of the striated muscles cells, as well as the shapes of the fat cells in the skin and the liver, looked similar to that of modern animals. However, neither neurons nor nerve tissues were present in the examined samples, and none of the “cells” that had retained their membranes had preserved the organelles and nuclei inside. The studies of the heart and blood vessels revealed several types of blood cells that were identified as erythrocytes, leukocytes (Fig. 6C,D), and possibly thrombocytes. What stuck out where discoid-shaped erythrocytes with a dent in the center similar to the shapes found in living mammals. The internal structures of these cells have never been studied, but scientists assumed that despite the preservation of the natural shapes of the cells they most likely contain only unstructured matrix.
Such erythrocyte “ghosts” were also found in the “blood” or hemolyzed blood clots sampled from the Maly Lyakhovsky mammoth discovered in 2012 on the Maly Lyakhovsky Island in the East-Siberian Sea. The histological study of this mammoth is on-going, but the first claims of findings of preserved cytoplasm in adipose tissue and nuclei in muscle and leukocyte cells were already announced .
Microscopic studies of the 10,000 year old Yuribei mammoth, which was found on the Gydan Peninsula in Western Siberia in 1979, also yielded interesting results. The carcass appeared to retain the complete skeleton and large portions of the liver, pancreas, stomach, intestines, and kidney. Examination of the liver and intestinal mesentery revealed dark spots in the central part of the cells, which were interpreted as nuclei, nucleoli or chromatin going through gradual stages of deterioration.
The brains of Ice Age mammals
Among all types of soft tissues nervous tissues of the brain, spinal cord, and peripheral nerves have the fastest rate of deterioration. The nervous tissues studied on even relatively recent human mummies showed very few neurons with preserved microscopically identifiable structures. Therefore, expectations have been fairly low for finding any nerve tissues in the ancient carcasses from the Ice Age deposits. This far, brain “tissues”, or rather the brain matrices surrounded by the preserved, very tough meninges, have only been found in four mummies. Unstructured brain mass was found during necropsy in the cranium of the Dima baby mammoth and the Khatanga mammoth from the Taimyr Peninsula. Mummies that contained brain “tissue” as detected by computed tomography (CT) scans were the Yukagir mammoth and "Sasha" the baby woolly rhino (Fig. 7), which were recovered from the permafrost of northern Yakutia in 2001 - 2004. The shapeless brain mass had such poor preservation that none of the tissue could be investigated at the histological or cellular level.

The year 2010 brought a big surprise and became a first “breakthrough” in the studies of the brains of Ice Age mammals, when within the skull of the Yuka mammoth scientists recognized a complete, slightly shrunken, but clearly detectable brain (Fig. 8). The Yuka mammoth was not complete, but retained the hide with hair, the head bones and complete lower parts of the legs. It was about 6-8 years old and lived about 39,000 years ago. Yuka’s external brain morphology and internal structures were comprehensively studied using CT imaging techniques, which allowed scientists to visualize the brain and its various internal structures, such as the amygdala, corpus callosum, and cerebellum. In overall, the brain anatomy appeared to be very similar to that of the African elephant .

The 42,000 year old baby mammoth "Lyuba" found in the Western Siberian Arctic is the first mammoth that seems to have nerve tissue preserved at the cellular level, including a few cell nuclei, along with other very well preserved tissue, such as blood vessels, collagen fibers and muscles .

Getting to germ cells
The last seven years were especially fruitful in terms of discoveries of Ice Age mummies in Eastern Siberia. Among those found are the Lyuba baby mammoth (Fig.8), the Yukagir bison (Fig. 9A), the Yukagir horse (Fig. 9B), the Sasha baby rhino (Fig. 7), two newborn siblings of cave lion cubs named Uyan (Fig. 9C) and Dina, and a not yet excavated partial body of the “Abyi" mammoth (Fig. 9D).

Given the remarkable preservation of many of these preserved mummies, it should not be surprising that scientists would attempt to recover genetic information in the form of DNA. Initially, this was attempted by trying to grow preserved cells in culture, so as to make enough copies of the cell to try to sequence DNA. Cell cultivation was first attempted using tissue from Dima. Specifically, samples were taken from the skin, muscle, blood vessel wall, and the “blood” collected from the blood vessels. These attempts did not yield any positive results. Attempts to identify chromosomes and their structure from the bone marrow were also unsuccessful, due to the failure of finding preserved cells or preserved cell nuclei.
The germ cells, egg and sperm cells that are produced by a mature animal, are extremely vulnerable and have one of the fastest rates of deterioration in the body upon its death. The search for egg or sperm cells from the frozen Ice Age mummies began in the late 1990s, soon after the successful cloning of "Dolly" the sheep in 1996. When the carcass of the 48,000 year old Zhenya mammoth bull was found in the Arctic of Western Siberia in 2012, which had a large portion of the hide, fat deposits and some other soft tissues preserved, scientists first thought to now have an ideal candidate for recovering germ cells. A fragment of the heart and most of his penis were recovered (Fig. 10). However, the advanced geological age and scavenging of the body by predators during the summer seasons prior to Zhenya’s burial did not bode well for sufficiently well preserved tissue. Indeed, investigation of the heart tissue showed heavy deterioration of the muscle cells and their membranes and the complete absence of internal cellular and nuclear structures .
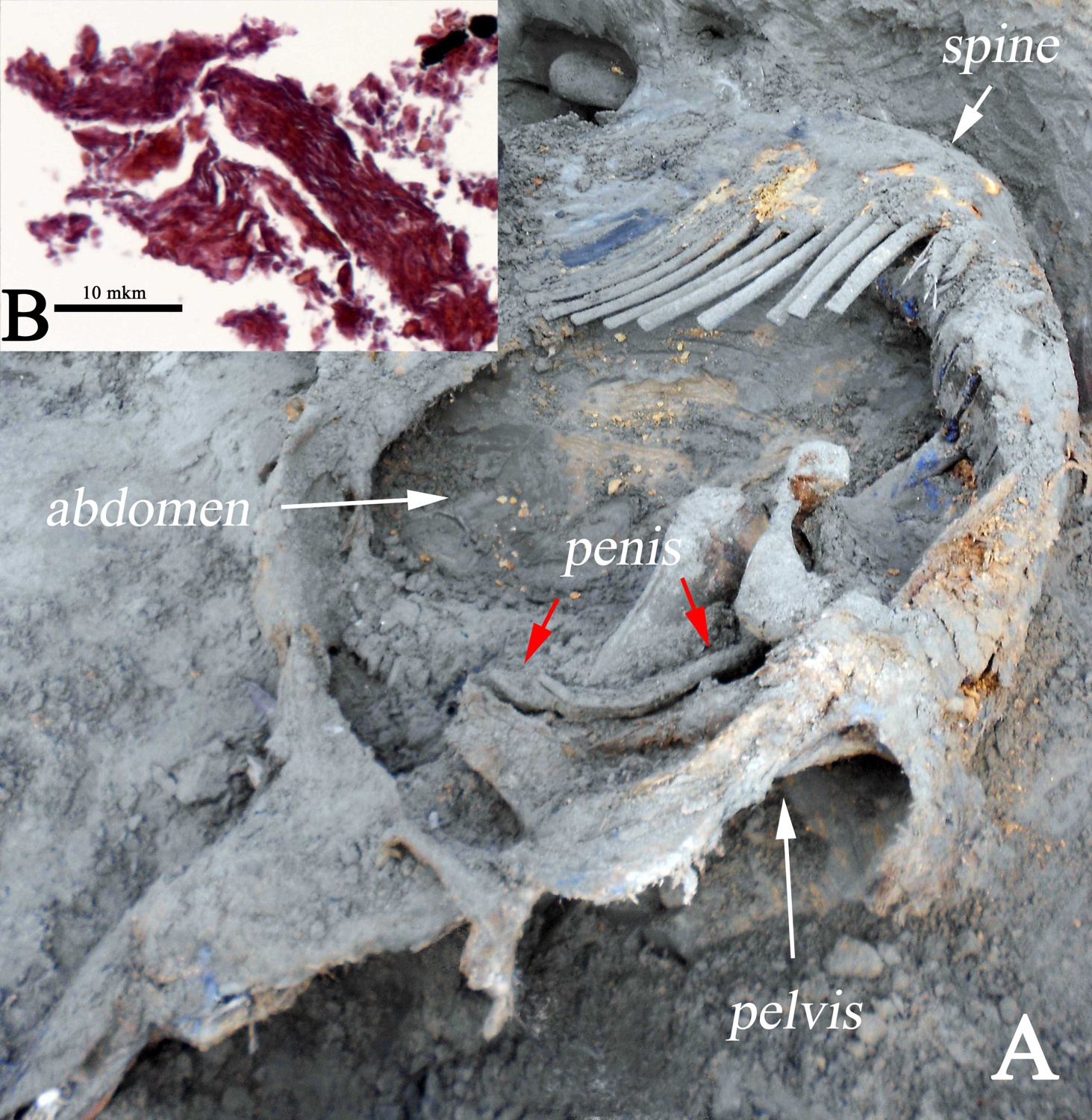
The penis was the only preserved organ of Zhenya’s reproductive system, and this mammoth became the fourth woolly mammoth mummy with this organ preserved. Among males, the Berezovka mammoth, the Bolshoi Lyakhovsky mammoth, and Dima were discovered with preserved penises and even with a testicle (Dima), but these were not appropriately preserved later on, or have never been investigated in a microscopic study. Zhenya’s penis was significantly deteriorated and has still not been examined microscopically.
Very few frozen carcasses reportedly preserved female reproductive organs. Among those that are preserved include a young, unnamed woolly rhino female found in the vicinity of the 39,000 year old Kolyma (Phillipova) woolly rhino. The unnamed female was found in 2012 in the lower Kolyma River basin. It had the vaginal area preserved, but the tissue has not yet been studied.
First attempts to extract ancient DNA
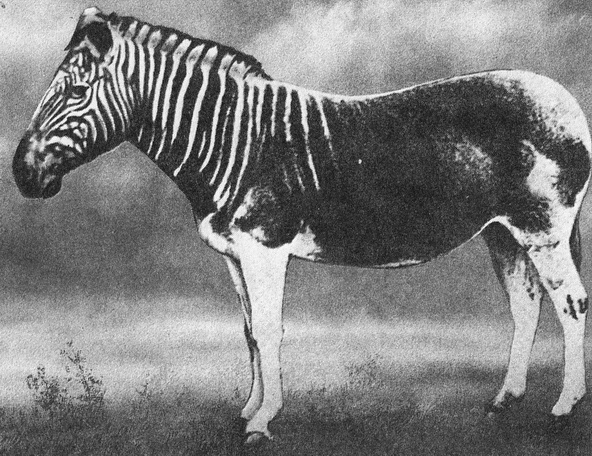
During the early 1980s, a team of scientists working in Allan Wilson’s lab at the University of California, Berkeley, recovered DNA fragments from a museum-preserved specimen of an extinct quagga, which is a close relative of the zebra (Fig. 11). This project demonstrated in principle that DNA can survive in some specimens after death, even when cells are degraded . When this result was published, excitement resurfaced that DNA could someday be recovered from mummified Ice Age animals and, perhaps, from plants and animals that lived millions of years ago.
Over the next decade, scientific papers were published in which researchers reported to have recovered DNA from extremely old remains, including DNA from dinosaur bones, Miocene-aged leaf fossils, and insects preserved in amber. Unfortunately, all of these claims of extremely ancient DNA have since been proven to be inauthentic; contamination of DNA sequences from the present-day environment is prolific, and all of these purportedly very old DNA sequences were actually contaminant DNA sequences from living organisms.
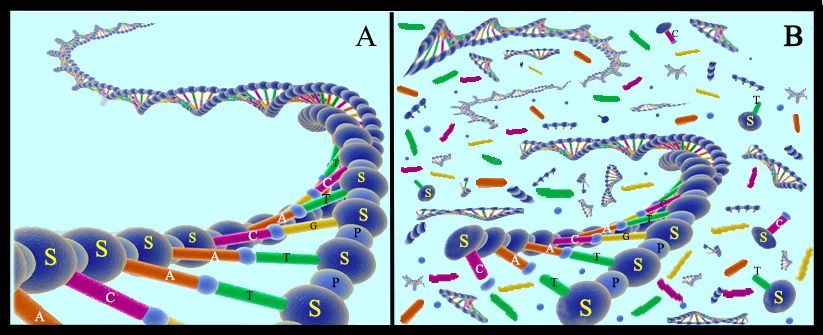
Since these early days of the scientific field called “ancient DNA,” the community of scientists aiming to recover DNA from the remains of organisms that used to be alive have developed improved techniques to extract DNA, and increasingly sophisticated techniques to discriminate authentic ancient DNA from contaminants. In addition to showing how easily an ancient sample can become contaminated with present-day DNA, this research has revealed many aspects of DNA survival and decay. For example, it is now known that the main first stage of DNA decay is by microbes that colonize decaying bodies that consume the DNA for energy. These microbes chop DNA molecules into increasingly small fragments, until fragments are too short to be useful. Chemicals such as water and oxygen, as well as ultraviolet radiation from the sun, also damage the DNA strands, causing the molecules to decay (Fig. 12). Given these processes, the geologically younger that the specimen is, the more likely it is that its DNA will be preserved. Scientists also learned that these decay processes are slowed in cold environments compared to hot environments, and in dry environments compared to wet environments. While all of these facts are good news for recovering DNA from the remains of Ice Age animals preserved in today’s cold regions, they do suggest a time limit for DNA survival. To date, the oldest DNA that has been recovered is from a horse bone that was collected from 700,000 year old dirt in Canada’s Yukon Territory . This bone had been frozen in the frozen dirt since the animal died, which probably explains its exceptional preservation.
Mummified remains, because of their appearance of exceptional preservation, have long been a favorite target of scientists working in ancient DNA. Some of the very first ancient DNA projects focused on recovering DNA from mummies, including from humans mummies from Egypt and the preserved mummified remains of mammoths. However, scientists quickly discovered that the soft tissues of mummies, despite their outward appearance of excellent preservation, tended not to be as well preserved from a genetic perspective as harder elements, such as bones, teeth, and hair. Although it is still not clear exactly why this is true, one explanation is that bones, once defleshed by predators, can be buried and frozen solidly more quickly than complete bodies, which limits the amount of time that the elements are exposed to microbial decay.
Challenges and pitfalls in extracting ancient DNA
Importantly, DNA preservation and the amount of contamination of DNA from living organisms vary considerably between samples. Both of these factors are important in determining how useful a particular sample will be for genetic analysis. In estimating DNA preservation, a researcher will ask whether ancient DNA is preserved, how much DNA is preserved, and how long the preserved DNA fragments are. If no DNA is preserved, the sample will not be useful for genetic analysis. In some samples, enough DNA is preserved to identify the species of the sample, but the extracted DNA is not complex, meaning that not enough molecules are preserved per unit of ground of bone or tissue to assemble a complete genome, for example, which may limit the utility of the sample for genetic analysis. If only very short DNA fragments are preserved, these fragments will be more difficult to use in assembling genomes: the shortest fragments that can be used to assemble genomes are in the range of 30 bases long, because the genomic location of molecules that are shorter than this can often not be identified with confidence. While molecules that are many millions of bases long can be extracted from modern samples, most ancient samples have average fragment lengths in the range of 20 bases to 100 bases. Finally, another important factor in determining whether a sample is useful for genetic analysis is the amount of contamination (by DNA from living organisms) that is in the sample. When DNA is extracted from an ancient bone or tissue sample, only some of the extracted DNA will belong to the organism whose remains are being studied. Most often, the majority of the extracted DNA will be from microbes, fungi, and other organisms that have infiltrated the sample since it was deposited in the ground. The proportion of extracted DNA that is from the host is called the endogenous DNA fraction, while the proportion of DNA originating from contaminants is called the exogenous fraction. Ancient samples vary in endogenous content from less than 1% endogenous DNA to more than 75% endogenous DNA. The greater the proportion of extracted DNA that belongs to the host, the fewer DNA sequences will need to be generated in order to have the same genomic coverage. Together, all of these factors: DNA fragment length, complexity of extracted molecules, and endogenous fraction, will determine what questions the sample will be useful to address. Ancient samples also vary considerably in their states of preservation and contamination, even samples of similar ages and from similar locations. In most cases, given sufficient sample, money, and time, even samples with low endogenous content and relatively short molecules can be used to assemble complete genome sequences: the scientists will simply need to use more samples and generate more sequence data from the most poorly preserved specimens than from the better preserved specimens. Because of these constraints, many genome sequences from ancient remains are incomplete: too little sample was available for destruction or the cost of sequencing would have been in excess of the budget available to generate a high-coverage, complete genome.
Ancient DNA from Ice Age mummies
Despite these constraints, ancient DNA has been recovered from many of the well preserved mummies described above, including the Yukagir mammoth, Yukagir bison, Uyan cave lion cub, Zhenya mammoth and Sasha rhino. While few of these have been sufficiently well preserved to generate high coverage complete genome sequences, recovered DNA from mummies has nonetheless been useful to better understand the evolutionary relationships between living and extinct species, such as between elephants and mammoths, and to predict changes in the genetic diversity and distribution of extinct species over time. DNA has also been recovered and studied from mummies found in locations across the globe outside of the Arctic. For example, DNA from Egyptian mummies, from naturally formed human mummies from high altitude caves in South America, and from the Tyrolean Iceman–a mummified human found in the mountains of central Europe who lived during the Neolithic-Copper Age transition–has shed light on early human migrations, about local adaptations to different environments, and about human cultural evolution. Importantly, not every question requires a high quality genome in order to be answered.
As the technologies to recover and sequence ancient DNA have continued to improve, DNA from mummies has been used not only to generate sequence data for the mummies themselves, but also to sequence DNA from the contents of their intestines, from swabs from their skins, and from the dirt that surrounds the buried remains. These DNA data have been used to reconstruct and describe the diet of extinct species, to understand the habitat in which they lived and the season in which they died, and to explore the community of microbes that lived in their guts. In this way, the DNA within mummified remains, while perhaps more poorly preserved than recovered from harder elements such as bones and teeth, can provide many more details about the lives, and deaths, of extinct species.
Attempts to resurrect a mammoth
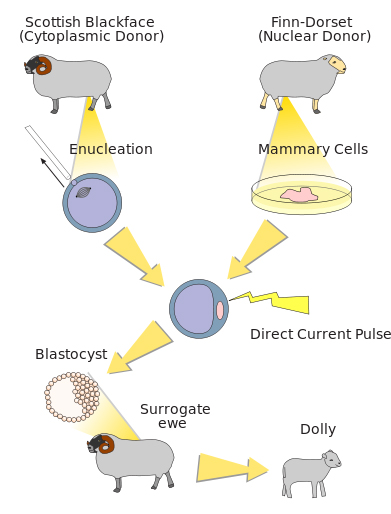
The exceptional preservation of many of the Ice Age mummies, combined with improved technologies to isolate genetic information from these mummies, have inspired many people to wonder whether–or when–it might become possible to use these mummies to bring extinct species back to life. This idea, which has been coined “de-extinction,” is captivating, but many hurdles remain for de-extinction to become reality. Several scientific teams are actively working on projects to resurrect a mammoth, although these teams are using different approaches to do so. A team led by Dr. WuSuk Huang, of Sooam Biotech in South Korea, hopes to find intact and living cells from preserved mammoth mummies that can be used to create a mammoth clone. His idea is to use the same approach, called somatic cell nuclear transfer, that allowed the birth of "Dolly" the sheep in 1996 (Fig. 13). To create Dolly, a team of scientists from the Roslin Institute in Scotland inserted the nucleus from a living sheep’s breast tissue cell into an unfertilized egg cell from a different sheep, which had had its own genetic material removed. The proteins in the egg cell caused the breast tissue nucleus to “reset,” and to direct the cell to divide and develop into all of the different cell types that make up an animal. After the embryo developed to a sufficient stage, it was transferred to a maternal host, where it continued to develop until birth. To translate this to cloning a mammoth, the nucleus from a living mammoth tissue cell would be transferred into an egg cell from an elephant and, after it began to develop, transferred into a surrogate elephant maternal host.
While cloning has been an increasingly successful tool, in particular for livestock animals, the process requires that an intact cell’s nucleus, with no genetic decay, is available from the animal that is to be cloned. Given what is known about preserved mammoth mummies and DNA decay–most mummies retain only very short DNA fragments (and certainly not living cells) and also have significant proportions of DNA that originate from contaminants rather than mammoths–the process of resurrecting a mammoth by cloning is not likely to be successful.
A team of scientists led by Dr. George Church at Harvard University are using a different approach to resurrect a mammoth, and their approach does not require any living mammoth cells. Instead, their approach takes advantage of the fact that, over the last few years, genome sequences have been assembled and published from several different mammoths, by several different research teams. To generate these genomes, scientists extracted DNA from bits of ground-up mammoth bone, and used high-throughput DNA sequencing technologies to sequences billions of fragments of short, damaged, ancient, mammoth DNA. Using computer algorithms, these scientists then mapped each of these short fragments of mammoth DNA to the already-sequenced genome of the Asian elephant, which is the closest living relative of mammoths. After these genomes were assembled, scientists scanned the genomes of mammoths and Asian elephants so that they could identify parts of the mammoth genome that were different from the Asian genome, with the idea that these parts of the genome were probably necessary to make a mammoth look and act like a mammoth, rather than like an elephant. Church’s team used this list of genetic differences between Asian elephants and mammoths to make a shortlist of important mammoth genes–genes that, if inserted into an elephant genome, could make that elephant look and act more like a mammoth. This team is currently using the genome-editing tool CRISPR/Cas to replace the elephant version of these genes with the mammoth version of these genes in elephant cells that are growing in their lab. Their plan is to use these living, edited, elephant cells (which now have some mammoth genes) as donor cells for a nuclear transfer into an enucleated elephant egg cell and grow an embryo, which they would then transfer to an elephant surrogate host. If that embryo developed fully and were born, it would be, genetically, part mammoth.
While mammoths and other extinct species are not likely ever to be cloned using frozen cells, genome editing approaches provide an interesting path forward to bringing extinct species, or at least many of their traits, back to life. Until that happens, however, scientists will continue to have new opportunities to learn about the life and history of these species, as new mummies are exposed by the melting permafrost of the northern Arctic.
Acknowledgements
The authors thank Elena Tikhonova (Zoological Institute, Russian Academy of Sciences, St. Petersburg) for providing photos (Fig. 3A, 6A-C,) from the ZIN archives. Julie Mossman (Hot Springs, USA) kindly edited the text.
Further reading on this topic:
- Mezrich B (2017) Woolly: The True Story of the Quest to Revive one of the History’s Most iconic Extinct Creatures. Atria Books
- Shapiro B (2015) How to Clone a Mammoth. The Science of De-Extinction. Princeton University Press
- Hagelberg E, Hofreiter M, and Keyser C (eds.) (2015) Ancient DNA: The First Three Decades. Phil. Trans. Roy. Soc. B 370: 20130371
- Shapiro B and Hofreiter M (2014) A Paleogenomic Perspective on Evolution and Gene Function: New Insights from Ancient DNA. Science 343: 1236573
- Lister A (2014) Mammoths and Mastodons. Firefly Books
- Lister A and Bahn P (2007) Mammoths: Giants of the Ice Age. University of California Press
- Stone R (2001) Mammoth: The Resurrection of an Ice Age Giant Perseus Publishing
- Guthrie D (1990) Frozen Fauna of the Mammoth Steppe: The Story of Blue Babe. University of Chicago Press
Responses